Benefits of Passivating
Stainless steel parts have a natural layer of oxide that provides limited protection for the parts. However, “free iron” can become attached to the part and overwhelm the oxide layer. The parts begin to rust and, eventually, lose their structural integrity.
Passivation both eliminates the free iron and strengthens the oxide layer, making the part more “passive”, that is less accepting of an assault by free iron. This increases the usable life of the part and reduces the likelihood of catastrophic failure and associated death and injuries. Passivation also significantly improves a part’s appearance. It also promotes the optimal flow of electricity.
F.M. Callahan & Son has specialized in passivating for over 50 years. Contact us today to discuss your passivating job.
There are different passivation processes for austenitic, martensitic, ferritic, and duplex stainless-steel alloys.
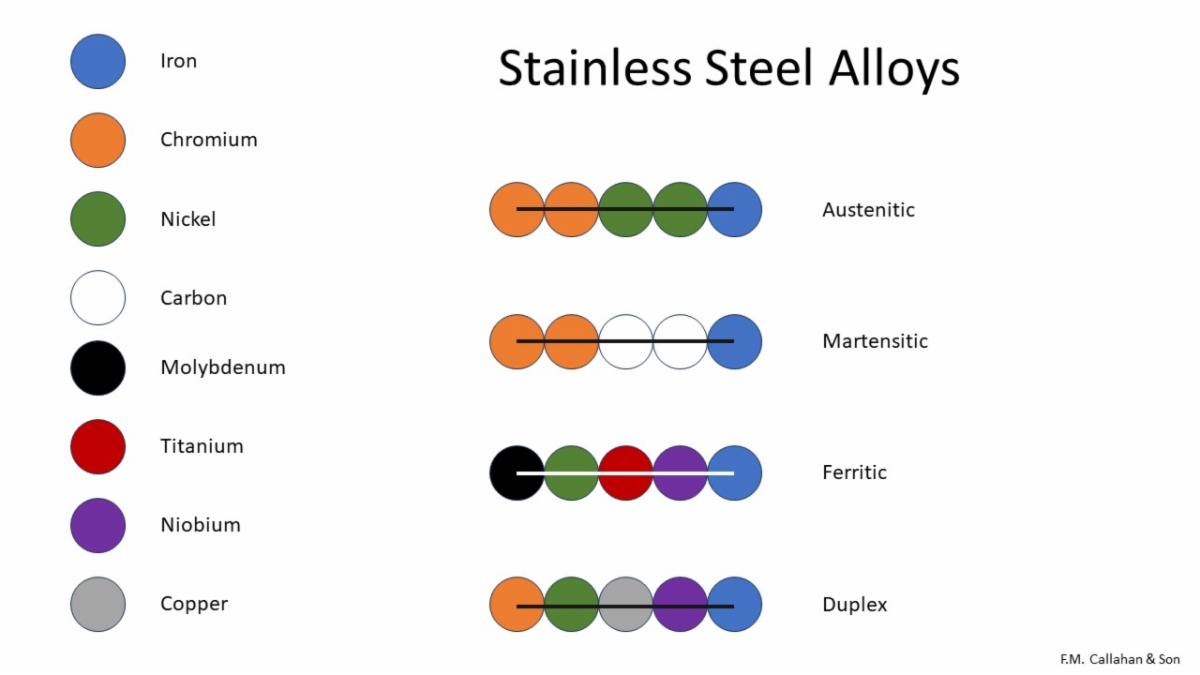
The figure above illustrates the four most common Stainless-Steel alloys and what the alloys are comprised of.
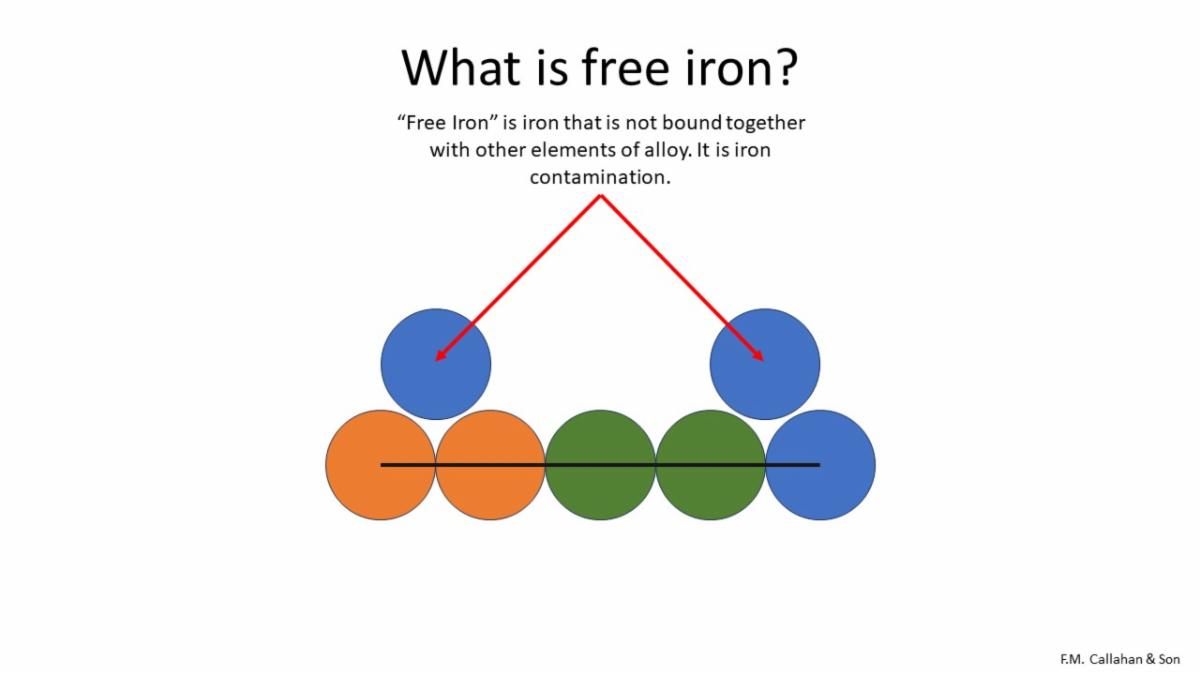
In the figure above, notice how the two free irons are not alloyed with the other elements.
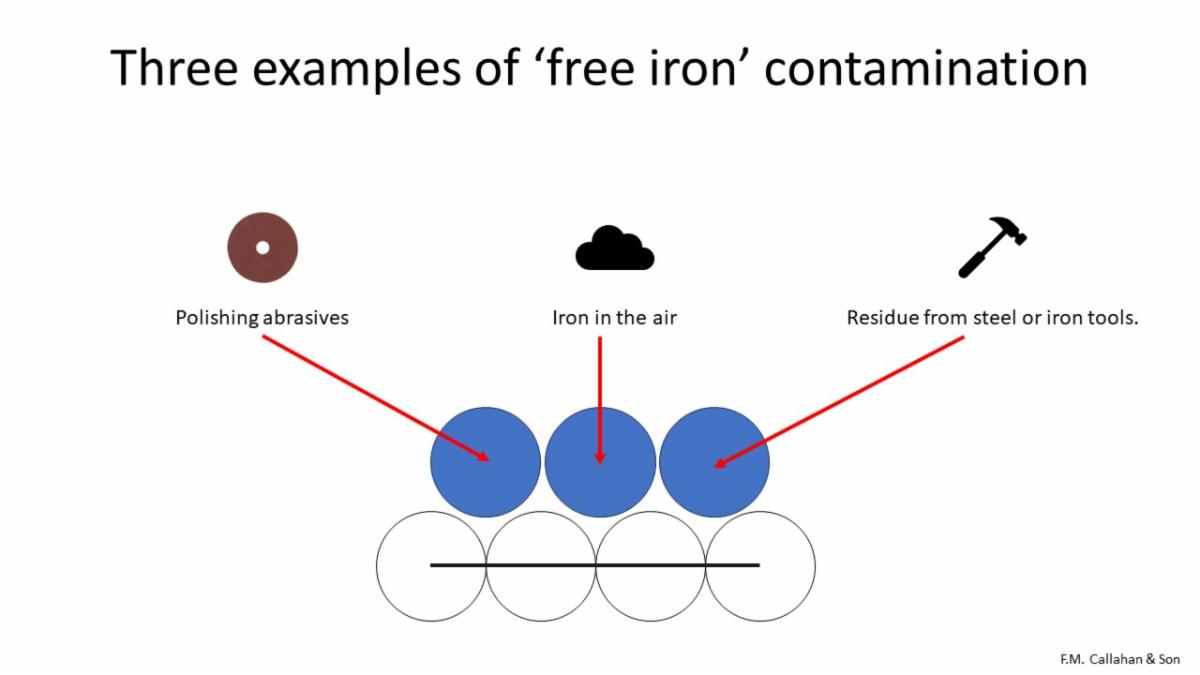
The figure above illustrates three of the many methods by which free ions are often deposited onto the surface of a part.
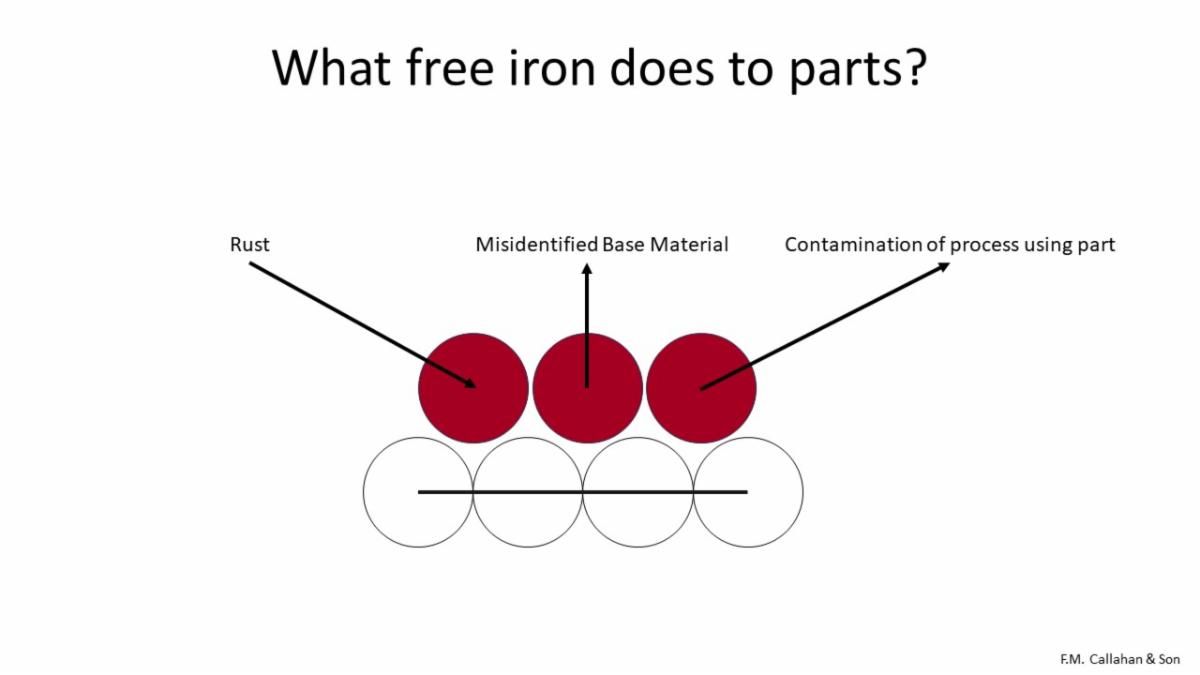
Free iron produces rust on a part. The rust can mislead people to think the part is steel when it is not. Free iron can also contaminate other processes.
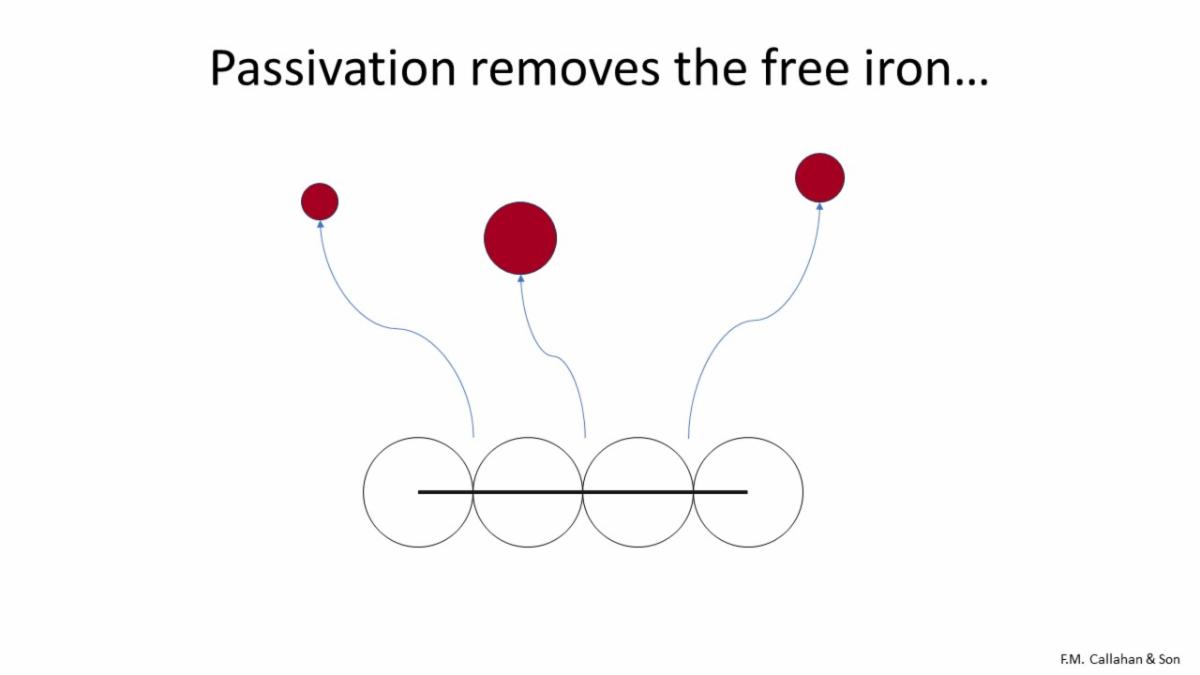
As illustrated above, the passivation process removes the free iron from the surface of the part.
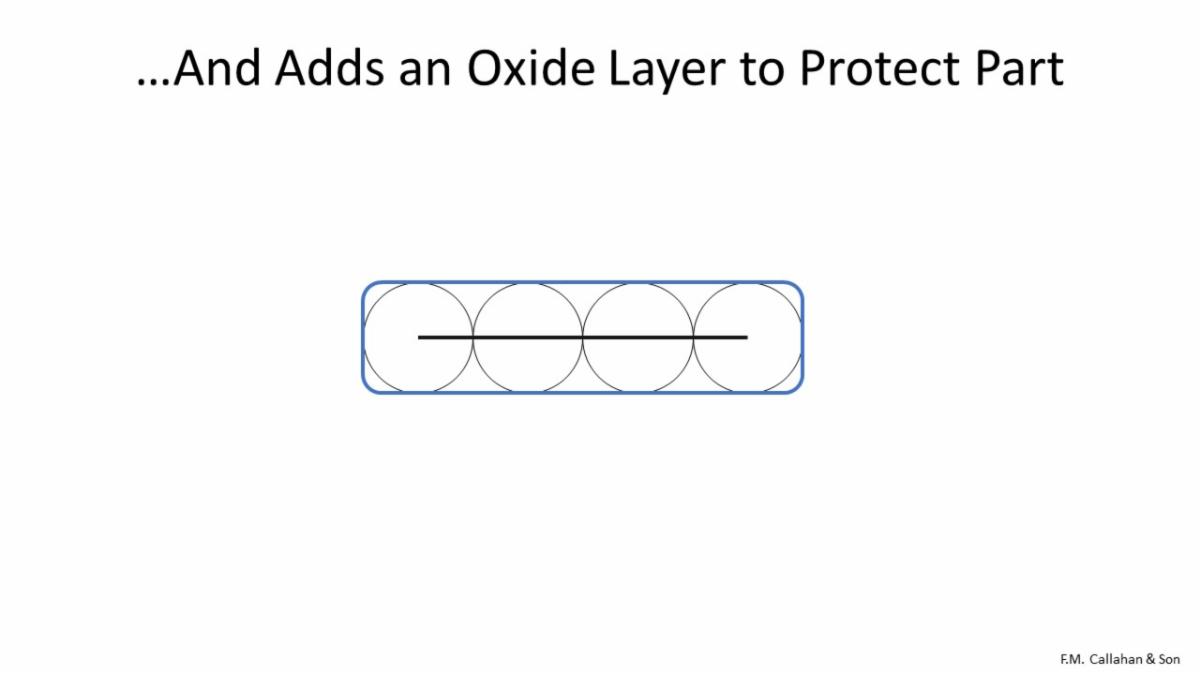
Passivation also puts a layer of oxide on the part to guard against corrosion.
F.M. Callahan & Son has been processing parts since 1910. Contact us today to discuss your passivating job.